Introduction
Silicon-based anode material are becoming a niche next-generation anode material used in high-performance lithium-ion batteries.
Compared with traditional secondary batteries, high-performance rechargeable lithium-ion batteries (“LIB”) have significant advantages in terms of volume specific energy, mass specific energy, mass specific power, cycle life, and charge-discharge efficiency. Today LIBs are used throughout the modern economy in fields including new energy vehicles, energy storage systems, and consumer electronics.
With development of new energy vehicles, customers are demanding EV-Type LIB have higher and higher battery energy density. However, manufacturers are maintaining the premise of producing high-performance batteries with characteristics of long life, high safety, and low cost.
Anode material plays an essential role in electrochemical activities of lithium-ion batteries, especially cycle performance, charging rate, and energy density. Therefore, the electrochemical performance of a lithium-ion battery depends on the anode material.
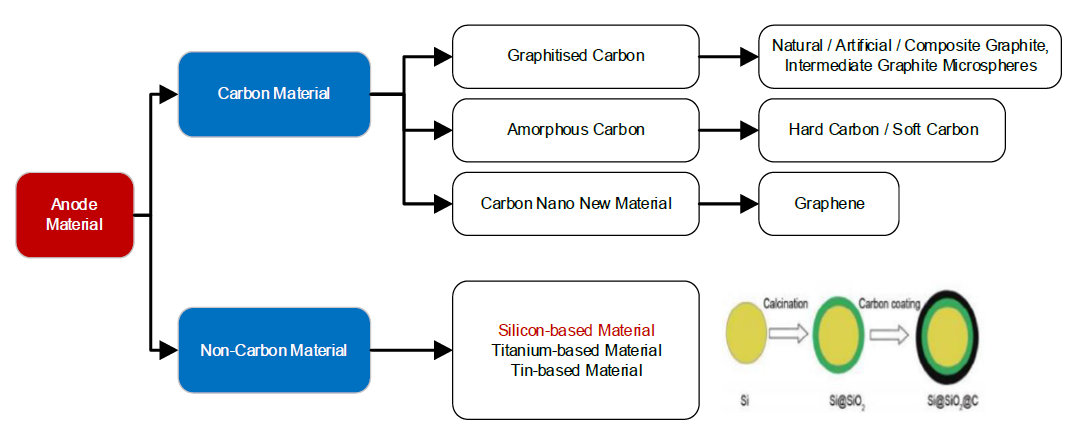
Development of anode material has been ongoing for more than 20 years, of which carbon-based materials are the most commercialised. Electrochemical activity of carbon material originates from the intercalation of lithium ions between graphene planes, which provides good mechanical properties, electrical conductivity, and lithium-ion transport.
In general, carbon materials balance the cost factor with good electrochemical activity. However, the low specific capacity of carbon material at 372 mAh g−1 cannot satisfy the demand of high energy density. It is this reason for the development of alternative anode materials in lithium-ion batteries.
Why Silicon is next-generation anode material
Among the anode materials of lithium-ion batteries, silicon-based materials such as pure silicon (Si), silicon monoxide (SiO), and silicon dioxide or silica (SiO2) are considered promising candidates for anodes of lithium-ion batteries.
Si-based materials have a relatively high theoretical capacity (1965 – 4200 mAh g−1). In general, Si-based materials have low electronic conductivity (10−1 S m−1), and the diffusion coefficient of lithium ion is also low (10−17 m2s−1).
Silicon (Si) has been considered as one of the best alternatives to replace graphite anodes for lithium-ion batteries owing to its high theoretical capacity (4200 mAh g−1), low discharge potential of 0.4 V (vs. Li/Li+), abundant availability, and environmental friendliness.
Silica (SiO2) has advantages such as a high theoretical capacity (1965 mAh g−1) and a low discharge potential 0.2 V (vs. Li/Li+). Early research indicated that SiO2 is electrochemically inactive when applied as a lithium-ion battery anode. In contrast, nano-SiO2 can react reversibly with lithium at low potentials. This has resulted in many studies on nano-SiO2 with various structures, such as thin films, nanotubes, nanocubes, and nanospheres.
Processing Methods
The most effective way to eliminate the negative effect from volume changes is to design efficient structures for silicon anodes to improve the structural stability of the silicon particles.
At present there are four principal production methods to produce silicon-based anode material namely
1) Mechanical ball milling method
2) chemical vapor deposition method
3) sol-gel method
4) high temperature pyrolysis method
Disadvantages of Silicon-Based Anode Material
The main two challenges when using silicon as the anode is first high-volume expansion and second being poor electrical conductivity.
- Poor cycle-life due to volumetric fluctuations during lithium ion intercalation and deintercalation,
- Irreversible capacity loss and low coulombic efficiency caused by mechanical fracture of Si anodes during alloying/dealloying process, and SEI issues.
However, despite challenges, the development of silicon-based anode materials is a promising area of research in advancing high-performance lithium-ion batteries.
Solutions include preparation of nanosilicon particles to alleviate the expansion of silicon and compounding with carbon giving silicon/carbon hybrid anodes. This is where silicon acts as the active material that provides high capacity and carbon improves the conductivity.
Competitive Landscape
Silicon-based anode material will become a niche product used high-energy density applications such as consumer electronics and electric vehicles.
In the field of EV-Type LIB, demand for high performance EVs has opened up the market for silicon-based anode material. Since 2021, several auto companies and lithium-ion battery companies have increased silicon-based anode material adoption.
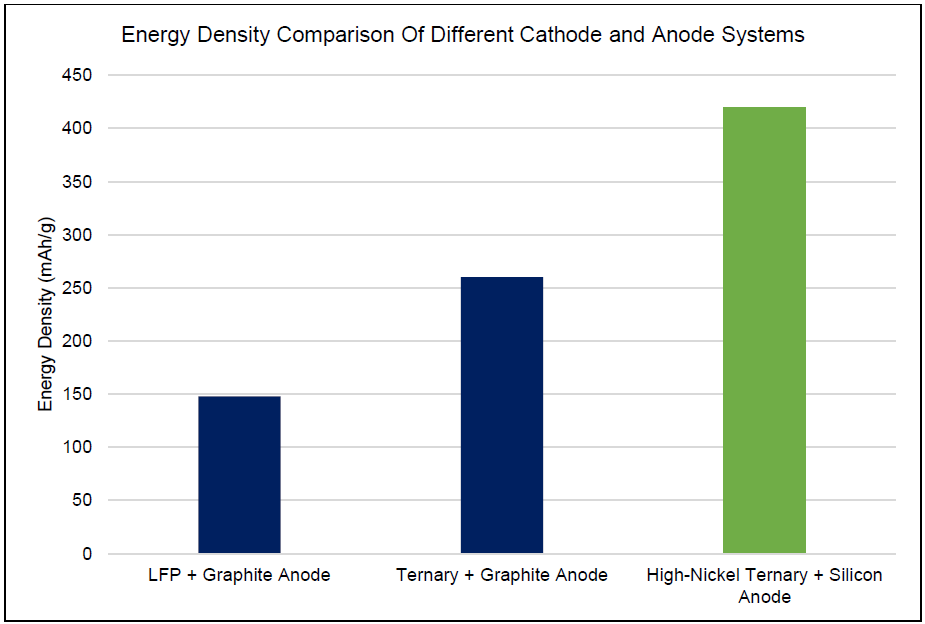
Tesla was the first to adopt silicon-containing anode material cylindrical batteries produced by Panasonic. Tesla applied silicon anode to its Model 3, adding 10% silicon to synthetic graphite, increasing the capacity of the anode to 550mAh/g, and the single energy density reaching 300Wh/kg.
In January 2022, Zhiji Automobile proposed to use “silicon-doped lithium supplement” technology in its EVs to achieve an energy density of 300Wh/kg and a 1000km cruising range.
NIO released pre-lithiated silicon carbon anode and belt a semi-solid battery with a power capacity of 150kWh and a cruising range of 1,000km, equipped with NIO ET7.
GAC AION LX uses silicon anode material battery technology, the specific energy of the cell exceeds 280Wh/kg and the battery life exceeds 1,000km.
In the future silicon-based anode material will become a niche anode material used in the lithium-ion battery sector applicable for high-energy density applications such as consumer electronics and electric vehicles.
Contact us to purchase the full report today
Click to view the Table of Contents

What is driving the growth of the silicon-based anode material industry?
1. Silicon has a specific capacity of 4,200 mAhg -1 and volume capacity of 9,786 mAh cm -3. This feature is the highest known for a lithium-ion battery anode material. Significantly higher than graphite-based anode material which has a limited theoretical specific capacity of ~370 mAh g −11.
2. Silicon is naturally abundant making it a sustainable for a raw material used in the production of anode material.
3. Silicon-based anode material promise longer-range, faster-charging, and more-affordable EVs. Silicon not only soaks up more lithium ions, but it also shuttles them across the battery’s membrane faster.